Implementación de un protocolo de bioprospección no invasivo para el aislamiento de Lactobacillus a partir de heces de gallinas en condiciones de forrajeo
Main Article Content
Keywords
Bioprospección, probióticos, bacterias ácido lácticas, prueba de antagonismo
Resumen
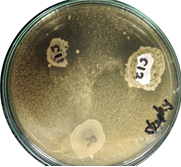
En la producción animal, los probióticos son una alternativa para reemplazar el uso de antibióticos, ya que disminuyen las tasas de morbilidad y mortalidad, aumentando al mismo tiempo la productividad. Así mismo, los probióticos constituyen una alternativa natural que, en contraste con los antibióticos, no generan patógenos resistentes ni dejan residuos químicos en el producto final. Una variedad de bacterias, pertenecientes al género Lactobacillus, han sido descritas como probióticos con alto potencial. Se estableció un protocolo de bioprospección no invasivo dirigido al aislamiento y caracterización de lactobacilos a partir de heces de gallinas. Las muestras fecales fueron colectadas a partir del suelo. Luego fueron diluidas y sembradas en medio selectivo para bacterias ácido lácticas MRS. Las colonias fueron identificadas por tres métodos: tinción de Gram, MALDI-TOF MS y secuenciamiento del gen ARNr 16s. Para caracterizar inicialmente el potencial probiótico de ocho de los aislados de lactobacilos obtenidos se realizaron pruebas de antagonismo usando cinco cepas referencia de patógenos: Staphylococcus aureus, Enterococcus faecium, Candida albicans, Pseudomonas spp. y Salmonella spp. 24 aislados distribuidos en cuatro especies de Lactobacillus fueron identificadas por MALDI-TOF MS. La identificación por MALDI-TOF MS fue confirmada mediante el secuenciamiento del gen ARNr 16s de ocho aislados seleccionados aleatoriamente. Cinco aislados, tres identificados como Lactobacillus plantarum y dos como Lactobacillus salivarius, inhibieron el crecimiento de al menos uno de los patógenos seleccionados. El protocolo logró un desempeño de 21 lactobacilos por 100 aislados, superando con creces la representación normal de los lacobacilos en el microbioma gastrointestinal de las gallinas, de manera que su implementación facilitaría los esfuerzos de aislamiento e identificación de nuevas cepas probióticas a partir de heces.
Descargas
Referencias
H. C. Wegener, “Antibiotics in animal feed and their role in resistance development,” Current opinion in microbiology, vol. 6, no. 5, pp. 439–445, 2003. [Online]. Available: https://doi.org/10.1016/j.mib.2003.09.009
F. M. Aarestrup, “Occurrence of glycopeptide resistance among enterococcus faecium isolates from conventional and ecological poultry farms,” Microbial Drug Resistance, vol. 1, no. 3, pp. 255–257, 1995. [Online]. Available: https://doi.org/10.1089/mdr.1995.1.255
J. Bates, J. Z. Jordens, and D. T. Griffiths, “Farm animals as a putative reservoir for vancomycin-resistant enterococcal infection in man,” Journal of Antimicrobial Chemotherapy, vol. 34, no. 4, pp. 507–514, 1994. [Online]. Available: https://doi.org/10.1093/jac/34.4.507
I. Klare, H. Heier, H. Claus, G. Böhme, S. Marin, G. Seltmann, R. Hakenbeck, V. Antanassova, and W. Witte, “Enterococcus faecium strains with vana-mediated high-level glycopeptide resistance isolated from animal foodstuffs and fecal samples of humans in the community,” Microbial Drug Resistance, vol. 1, no. 3, pp. 265–272, 1995.
J. Stephenson, “Antibiotics in animal feed,” JAMA, vol. 290, no. 11, pp. 1443–1443, 2003.
F. Guarner and G. Schaafsma, “Probiotics.” International journal of food microbiology, vol. 39, no. 3, pp. 237–238, 1998.
F. Chaucheyras-Durand and H. Durand, “Probiotics in animal nutrition and health,” Beneficial microbes, vol. 1, no. 1, pp. 3–9, 2009. [Online]. Available: https://doi.org/10.3920/BM2008.1002
M. Vanbelle, E. Teller, and M. Focant, “Probiotics in animal nutrition: a review,” Archives of Animal Nutrition, vol. 40, no. 7, pp. 543–567, 1990. [Online]. Available: https://doi.org/10.1080/17450399009428406
P. Banjeree and N. Pradhan, “Live yeasts a good alternative to agp in broiler chickens,” World Poultry, vol. 22, no. 8, pp. 32–34, 2006. [Online]. Available: https://www.poultryworld.net/PageFiles/27778/001_ boerderij-download-WP6926D01.pdf
J. Higgins, S. Higgins, J. Vicente, A. Wolfenden, G. Tellez, and B. Hargis, “Temporal effects of lactic acid bacteria probiotic culture on salmonella in neonatal broilers,” Poultry Science, vol. 86, no. 8, pp. 1662–1666, 2007. [Online]. Available: https://doi.org/10.1093/ps/86.8.1662
S. Higgins, J. Higgins, A. Wolfenden, S. Henderson, A. Torres-Rodriguez, G. Tellez, and B. Hargis, “Evaluation of a lactobacillus-based probiotic culture for the reduction of salmonella enteritidis in neonatal broiler chicks,” Poultry Science, vol. 87, no. 1, pp. 27–31, 2008. [Online]. Available: https://doi.org/10.3382/ps.2007-00210
R. La Ragione, A. Narbad, M. Gasson, and M. J. Woodward, “In vivo characterization of lactobacillus johnsonii fi9785 for use as a defined competitive exclusion agent against bacterial pathogens in poultry,” Letters in Applied Microbiology, vol. 38, no. 3, pp. 197–205, 2004.
R. M. La Ragione and M. J. Woodward, “Competitive exclusion by bacillus subtilis spores of salmonella enterica serotype enteritidis and clostridium perfringens in young chickens,” Veterinary microbiology, vol. 94, no. 3, pp. 245–256, 2003. [Online]. Available: https://doi.org/10.1016/S0378-1135(03) 00077-4
G. Tellez, C. Pixley, R. Wolfenden, S. Layton, and B. Hargis, “Probiotics/direct fed microbials for salmonella control in poultry,” Food Research International, vol. 45, no. 2, pp. 628–633, 2012. [Online]. Available: https://doi.org/10.1016/j.foodres.2011.03.047
F. Yan, W. Wang, R. Wolfenden, and H. Cheng, “The effect of bacillus subtilis based probiotic on bone health in broiler chickens,” Poultry Science, vol. 95, p. 40, 2016. [Online]. Available: https://doi.org/10.1093/jas/sky092
T. Inatomi, “Growth performance, gut mucosal immunity and carcass and intramuscular fat of broilers fed diet containing a combination of three probiotics,” Sci Postprint, vol. 1, p. e00052, 2015.
V. Kurtoglu*, F. Kurtoglu, E. Seker, B. Coskun, T. Balevi, and E. Polat, “Effect of probiotic supplementation on laying hen diets on yield performance and serum and egg yolk cholesterol,” Food additives and contaminants, vol. 21, no. 9, pp. 817–823, 2004. [Online]. Available: https://doi.org/10.1080/02652030310001639530
C. Pineda-Quiroga, R. Atxaerandio, I. Zubiria, I. Gonzalez-Pozuelo, A. Hurtado, R. Ruiz, and A. Garcia-Rodriguez, “Productive performance and cecal microbial counts of floor housed laying hens supplemented with dry whey powder alone or combined with pediococcus acidilactici in the late phase of production,” Livestock Science, vol. 195, pp. 9–12, 2017. [Online]. Available: https://doi.org/10.1016/j.livsci.2016.11.007
M. Yörük, M. Gül, A. Hayirli, and M. Macit, “The effects of supplementation of humate and probiotic on egg production and quality parameters during the late laying period in hens,” Poultry Science, vol. 83, no. 1, pp. 84–88, 2004. [Online]. Available: https://doi.org/10.1093/ps/83.1.84
R. Fuller, “Ecological studies on the lactobacillus flora associated with the crop epithelium of the fowl,” Journal of Applied Bacteriology, vol. 36, no. 1, pp. 131–139, 1973. [Online]. Available: https://doi.org/10.1111/j.1365-2672. 1973.tb04080.x
J. Patterson and K. Burkholder, “Application of prebiotics and probiotics in poultry production,” Poultry science, vol. 82, no. 4, pp. 627–631, 2003. [Online]. Available: https://doi.org/10.1093/ps/82.4.627
B. B. Oakley, H. S. Lillehoj, M. H. Kogut, W. K. Kim, J. J. Maurer, A. Pedroso, M. D. Lee, S. R. Collett, T. J. Johnson, and N. A. Cox, “The chicken gastrointestinal microbiome,” FEMS microbiology letters, vol. 360, no. 2, pp. 100–112, 2014. [Online]. Available: 10.1111/1574-6968.12608
P. J. Turnbaugh, V. K. Ridaura, J. J. Faith, F. E. Rey, R. Knight, and J. I. Gordon, “The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice,” Science translational medicine, vol. 1, no. 6, pp. 6ra14–6ra14, 2009. [Online]. Available: https://doi.org/10.1126/scitranslmed.3000322
E. Carbonnelle, C. Mesquita, E. Bille, N. Day, B. Dauphin, J.-L. Beretti, A. Ferroni, L. Gutmann, and X. Nassif, “Maldi-tof mass spectrometry tools for bacterial identification in clinical microbiology laboratory,” Clinical biochemistry, vol. 44, no. 1, pp. 104–109, 2011. [Online]. Available: https://doi.org/10.1016/j.clinbiochem.2010.06.017
J. M. Janda and S. L. Abbott, “16s rrna gene sequencing for bacterial identification in the diagnostic laboratory: pluses, perils, and pitfalls,” Journal of clinical microbiology, vol. 45, no. 9, pp. 2761–2764, 2007. [Online]. Available: https://doi.org/10.1128/JCM.01228-07
U. Schillinger and F. K. Lücke, Applied and environmental microbiology, vol. 55, no. 8, pp. 1901–1906, 1989.
T. A. Tatusova and T. L. Madden, “Blast 2 sequences, a new tool for comparing protein and nucleotide sequences,” FEMS microbiology letters, vol. 174, no. 2, pp. 247–250, 1999. [Online]. Available: https://doi.org/10.1016/S0378-1097(99)00149-4
K. Sonomoto and A. Yokota, Lactic acid bacteria and bifidobacteria: current progress in advanced research. Horizon Scientific Press, 2011.
S. C. Corr, Y. Li, C. U. Riedel, P. W. O’Toole, C. Hill, and C. G. Gahan, “Bacteriocin production as a mechanism for the antiinfective activity of lactobacillus salivarius ucc118,” Proceedings of the National Academy of Sciences, vol. 104, no. 18, pp. 7617–7621, 2007. [Online]. Available: https://doi.org/10.1073/pnas.0700440104
K. A. Ryan, P. Daly, Y. Li, C. Hooton, and P. W. O’Toole, “Strain-specific inhibition of helicobacter pylori by lactobacillus salivarius and other lactobacilli,” Journal of Antimicrobial Chemotherapy, vol. 61, no. 4, pp. 831–834, 2008. [Online]. Available: https://doi.org/10.1093/jac/dkn040
C. Dunne, L. Murphy, S. Flynn, L. O?Mahony, S. O?Halloran, M. Feeney, D. Morrissey, G. Thornton, G. Fitzgerald, C. Daly et al., “Probiotics: from myth to reality. demonstration of functionality in animal models of disease and in human clinical trials,” in Lactic Acid Bacteria: Genetics, Metabolism and Applications. Springer, 1999, pp. 279–292.
S. Messaoudi, M. Manai, G. Kergourlay, H. Prévost, N. Connil, J.-M. Chobert, and X. Dousset, “Lactobacillus salivarius: bacteriocin and probiotic activity,” Food microbiology, vol. 36, no. 2, pp. 296–304, 2013. [Online]. Available: https://doi.org/10.1016/j.fm.2013.05.010
M. Velraeds, H. Van der Mei, G. Reid, and H. J. Busscher, “Inhibition of initial adhesion of uropathogenic enterococcus faecalis by biosurfactants from lactobacillus isolates.” Applied and environmental microbiology, vol. 62, no. 6, pp. 1958–1963, 1996. [Online]. Available: https://doi.org/10.1016/S0090-4295(97)00065-4
C. M. Galdeano and G. Perdigon, “The probiotic bacterium lactobacillus casei induces activation of the gut mucosal immune system through innate immunity,” Clinical and Vaccine Immunology, vol. 13, no. 2, pp. 219–226, 2006. [Online]. Available: https://doi.org/10.1128/CVI.13.2.219-226.2006
G. Storelli, A. Defaye, B. Erkosar, P. Hols, J. Royet, and F. Leulier, “Lactobacillus plantarum promotes drosophila systemic growth by modulating hormonal signals through tor-dependent nutrient sensing,” Cell metabolism, vol. 14, no. 3, pp. 403–414, 2011. [Online]. Available: https://doi.org/10.1016/j.cmet.2011.07.012
F. L. Tulini, L. K. Winkelströter, and E. C. De Martinis, “Identification and evaluation of the probiotic potential of lactobacillus paraplantarum ft259, a bacteriocinogenic strain isolated from brazilian semi-hard artisanal cheese,” Anaerobe, vol. 22, pp. 57–63, 2013. [Online]. Available: https://doi.org/10.1016/j.anaerobe.2013.06.006