Determinación de parámetros cinéticos en la biosorción de Cromo (VI) en solución acuosa
Main Article Content
Keywords
Modelos cinéticos, ion metálico, cáscaras, remoción, Cromo (VI)
Resumen
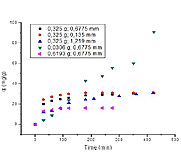
La contaminación de cuerpos acuáticos por metales pesados es un problema ambiental creciente haciendo cada vez más importante el estudio y desarrollo de nuevas tecnologías y materiales que puedan ser usados para la remoción de este tipo de contaminantes. Así surge la adsorción usando materiales residuales como una alternativa sostenible para la solución de esta problemática. En el presente estudio se propone el uso de las cáscaras de plátano en la adsorción de Cr (VI) en un sistema por lotes estableciendo la cinética del proceso a diferentes condiciones de temperatura, tamaño de partícula y cantidad de adsorbente. El ajuste de los datos fue hecho usando los modelos teóricos de pseudo-primer orden, pseudo-segundo orden y Elovich. De los datos se establece que son los modelos de pesudo-segundo orden y Elovich los que muestran un mejor ajuste determinado así que la adsorción en el material se da sobre dos sitios de adsorción y que tal proceso está relacionado con una adsorción química. La máxima capacidad de adsorción de Cr (VI) fue encontrada a una condición de 0.0306 g, 0.6775 mm y 55°C a un tiempo de 420 min estableciendo el uso eficiente de cáscaras de plátano para la remoción del ion metálico en estudio.
Descargas
Referencias
[1] F. Mutongo, O. Kuipa, and P. K. Kuipa, “Removal of cr (vi) from aqueous solutions using powder of potato peelings as a low cost sorbent,” Bioinorganic chemistry and applications, vol. 2014, 2014. https://doi.org/10.1155/2014/973153
[2] A. Rosales, C. Rodríguez, and M. Ballen-Segura, “Remoción de contaminantes y crecimiento del alga scenedesmus sp. en aguas residuales de curtiembres, comparación entre células libres e inmovilizadas,”. Ingeniería y Ciencia, vol. 14, no. 28, pp. 11–34, 2018. https://doi.org/10.17230/ingciencia.14.28.1
[3] S. A. Sadeek, N. A. Negm, H. H. Hefni, and M. M. A. Wahab, “Metal adsorption by agricultural biosorbents: Adsorption isotherm, kinetic and biosorbents chemical structures,” International Journal of iological Macromolecules, vol. 81, pp. 400 – 409, 2015. https://doi.org/10.1016/j.ijbiomac.2015.08.031
[4] M. Akram, H. N. Bhatti, M. Iqbal, S. Noreen, and S. Sadaf, “Biocomposite efficiency for cr(vi) adsorption: Kinetic, equilibrium and thermodynamics studies,” Journal of Environmental Chemical Engineering, vol. 5, no. 1, pp. 400 – 411, 2017. https://doi.org/10.1016/j.jece.2016.12.002
[5] E. Agrafioti, D. Kalderis, and E. Diamadopoulos, “Arsenic and chromium removal from water using biochars derived from rice husk, organic solid wastes and sewage sludge,” Journal of Environmental Management, vol. 133, pp. 309 – 314, 2014. 131
[6] N. K. Mondal, A. Samanta, S. Chakraborty, and W. A. Shaikh, “Enhanced chromium (vi) removal using banana peel dust: isotherms, kinetics and thermodynamics study,” Sustainable Water Resources Management, vol. 4, no. 3, pp. 489–497, 2018. https://doi.org/10.1007/s40899-017-0130-7
[7] S. Mishra and R. N. Bharagava, “Toxic and genotoxic effects of hexavalent chromium in environment and its bioremediation strategies,” Journal of Environmental Science and Health, Part C, vol. 34, no. 1, pp. 1–32, 2016, pMID: 26398402. https://doi.org/10.1080/10590501.2015.1096883
[8] J. Silva, A. Paiva, D. Soares, A. Labrincha, and F. Castro, “Solvent extraction applied to the recovery of heavy metals from galvanic sludge,” Journal of Hazardous Materials, vol. 120, no. 1, pp. 113 – 118, 2005. https://doi.org/10.1016/j.jhazmat.2004.12.008
[9] Z. Chen, Y. Liang, D. Jia, W. Chen, Z. Cui, and X. Wang, “Layered silicate rub-15 for efficient removal of uo 2 2+ and heavy metal ions by ion-exchange,” Environmental Science: Nano, vol. 4, no. 9, pp. 1851–1858, 2017. https://doi.org/10.1039/C7EN00366H
[10] A. Azimi, A. Azari, M. Rezakazemi, and M. Ansarpour, “Removal of heavy metals from industrial wastewaters: a review,” ChemBioEng Reviews, vol. 4, no. 1, pp. 37–59, 2017. https://doi.org/10.1002/cben.201600010
[11] S. Bolisetty, M. Peydayesh, and R. Mezzenga, “Sustainable technologies for water purification from heavy metals: review and analysis,” Chemical Society Reviews, vol. 48, no. 2, pp. 463–487, 2019. https://doi.org/10.1039/C8CS00493E
[12] C. F. Carolin, P. S. Kumar, A. Saravanan, G. J. Joshiba, and M. Naushad, “Efficient techniques for the removal of toxic heavy metals from aquatic environment: A review,” Journal of Environmental Chemical Engineering, vol. 5, no. 3, pp. 2782 – 2799, 2017.
[13] Y. Li, J. Liu, Q. Yuan, H. Tang, F. Yu, and X. Lv, “A green adsorbent derived from banana peel for highly effective removal of heavy metal ions from water,” Rsc Advances, vol. 6, no. 51, pp. 45 041–45 048, 2016.
https://doi.org/10.1039/C6RA07460J
[14] M. Vasudevan, P. Ajithkumar, R. Singh, and N. Natarajan, “Mass transfer kinetics using two-site interface model for removal of cr (vi) from aqueous solution with cassava peel and rubber tree bark as adsorbents,” Environmental Engineering Research, vol. 21, no. 2, pp. 152–163, 2016. https://doi.org/10.4491/eer.2015.152
[15] R. Naik, S. Ratan, and I. Singh, “Use of orange peel as an adsorbent for the removal of cr(vi) from its aqueous solution,” Indian Journal of Chemical Technology, vol. 25, pp. 300–305, 05 2018.
[16] C. Tejada-Tovar, A. Villabona-Ortíz, Á. D. González-Delgado, C. Granados-Conde, and M. Jiménez-Villadiego, “Kinetics of mercury and nickel adsorption using chemically pretreated cocoa (theobroma cacao)
husks,” Transactions of the ASABE, vol. 62, no. 2, pp. 461–466, 2019.
[17] M. Nigam, S. Rajoriya, S. R. Singh, and P. Kumar, “Adsorption of cr (vi) ion from tannery wastewater on tea waste: kinetics, equilibrium and thermodynamics studies,” Journal of Environmental Chemical Engineering, vol. 7, no. 3, p. 103188, 2019. https://doi.org/10.1016/j.jece.2019.103188
[18] N. M. Rane, S. V. Admane, and R. S. Sapkal, “Adsorption of hexavalent chromium from wastewater by using sweetlime and lemon peel powder by batch studies,” in Waste Management and Resource Efficiency, S. K. Ghosh, Ed. Singapore: Springer Singapore, 2019, pp. 1207–1220.
[19] D. L. Gómez Aguilar, J. P. Rodríguez Miranda, E. Muñoz, J. Andrés, P. Betancur, J. Fredy et al., “Coffee pulp: A sustainable alternative removal of cr (vi) in wastewaters,” Processes, vol. 7, no. 7, p. 403, 2019.
https://doi.org/10.3390/pr7070403
[20] I. Enniya, L. Rghioui, and A. Jourani, “Adsorption of hexavalent chromium in aqueous solution on activated carbon prepared from apple peels,” Sustainable Chemistry and Pharmacy, vol. 7, pp. 9–16, 2018.
https://doi.org/10.1016/j.scp.2017.11.003
[21] M. Ahmadi, E. Kouhgardi, and B. Ramavandi, “Physico-chemical study of dew melon peel biochar for chromium attenuation from simulated and actual wastewaters,” Korean Journal of Chemical Engineering, vol. 33, no. 9, pp. 2589–2601, 2016. https://doi.org/10.1007/s11814-016-0135-1
[22] G. A. El-Din, A. Amer, G. Malsh, and M. Hussein, “Study on the use of banana peels for oil spill removal,” Alexandria engineering journal, vol. 57, no. 3, pp. 2061–2068, 2018. https://doi.org/10.1016/j.aej.2017.05.020
[23] J. Núñez-Zarur, C. Tejada-Tovar, A. Villabona-Ortíz, D. Acevedo, and R. Tejada-Tovar, “Thermodynamics, kinetics and equilibrium adsorption of cr (vi) and hg (ii) in aqueous solution on corn husk (zea mays),”
International Journal of ChemTech Research, vol. 11, no. 5, pp. 265–280, 2018. http://dx.doi.org/10.20902/IJCTR.2018.110529
[24] C. Tejada-Tovar, A. Herrera-Barros, and A. Villabona-Ortíz, “Assessment of chemically modified lignocellulose waste for the adsorption of cr (vi),” Revista Facultad de Ingeniería, vol. 29, no. 54, pp. e10 298–e10 298, 2020. https://doi.org/10.19053/01211129.v29.n54.2020.10298
[25] Y. A. Neolaka, G. Supriyanto, and H. S. Kusuma, “Adsorption performance of cr (vi)-imprinted poly (4-vp-co-mma) supported on activated Indonesia (ende-flores) natural zeolite structure for cr (vi) removal from aqueous solution,” Journal of Environmental Chemical Engineering, vol. 6, no. 2, pp. 3436–3443, 2018. https://doi.org/10.1016/j.jece.2018.04.053
[26] C. Tien and B. V. Ramarao, “On the significance and utility of the lagergren model and the pseudo second-order model of batch adsorption,” Separation Science and Technology, vol. 52, no. 6, pp. 975–986, 2017. https://doi.org/10.1080/01496395.2016.1274327
[27] Y.-S. Ho and G. McKay, “Pseudo-second order model for sorption processes,” Process biochemistry, vol. 34, no. 5, pp. 451–465, 1999. https://doi.org/10.1016/S0032-9592(98)00112-5
[28] M. Low, “Kinetics of chemisorption of gases on solids,” Chemical Reviews, vol. 60, no. 3, pp. 267–312, 1960.
[29] X. Song, L. Li, Z. Geng, L. Zhou, and L. Ji, “Effective and selective adsorption of as (iii) via imprinted magnetic fe3o4/htcc composite nanoparticles,” Journal of environmental chemical engineering, vol. 5, no. 1,
pp. 16–25, 2017. https://doi.org/10.1021/cr60205a003
[30] X. Tao, Y. Wu, and L. Cha, “Shaddock peels-based activated carbon as cost-saving adsorbents for efficient removal of cr (vi) and methyl orange,” Environmental Science and Pollution Research, vol. 26, no. 19, pp. 19 828–19 842, 2019. https://doi.org/10.1007/s11356-019-05322-8
[31] E.-K. Guechi and O. Hamdaoui, “Evaluation of potato peel as a novel adsorbent for the removal of cu (ii) from aqueous solutions: equilibrium, kinetic, and thermodynamic studies,” Desalination and Water Treatment, vol. 57, no. 23, pp. 10 677–10 688, 2016. https://doi.org/10.1080/19443994.2015.1038739
[32] G. Adebayo, A. Mohammed, and S. Sokoya, “Biosorption of fe (ii) and cd (ii) ions from aqueous solution using a low cost adsorbent from orange peels,” Journal of Applied Sciences and Environmental Management, vol. 20, no. 3, pp. 702–714, 2016. https://doi.org/10.4314/jasem.v20i3.25
[33] P. Premkumar, S. Ramasamy et al., “Comparative studies on the removal of chromium (vi) from aqueous solutions using raw and modified citrus limettioides peel,” Indian Journal of Chemical Technology (IJCT), vol. 25, no. 3, pp. 255–265, 2018.
[34] X. Li, K. Wang, and Y. Peng, “Exploring the interaction of silver nanoparticles with pepsin and its adsorption isotherms and kinetics,” Chemico-biological interactions, vol. 286, pp. 52–59, 2018. https://doi.org/10.1016/j.cbi.2018.03.004
[35] D. C. Ong, S. M. B. Pingul-Ong, C.-C. Kan, and M. D. G. de Luna, “Removal of nickel ions from aqueous solutions by manganese dioxide derived from groundwater treatment sludge,” Journal of cleaner production, vol. 190, pp. 443–451, 2018. https://doi.org/10.1016/j.jclepro.2018.04.175
[36] M. O. Borna, M. Pirsaheb, M. V. Niri, R. K. Mashizie, B. Kakavandi, M. R. Zare, and A. Asadi, “Batch and column studies for the adsorption of chromium (vi) on low-cost hibiscus cannabinus kenaf, a green adsorbent,” Journal of the Taiwan Institute of Chemical Engineers, vol. 68, pp. 80–89, 2016. https://doi.org/10.1016/j.jtice.2016.09.022
[37] S. Parlayici and E. Pehlivan, “Comparative study of cr (vi) removal by bio-waste adsorbents: equilibrium, kinetics, and thermodynamic,” Journal of Analytical Science and Technology, vol. 10, no. 1, pp. 1–8, 2019.
https://doi.org/10.1186/s40543-019-0175-3
[38] M. Manjuladevi, R. Anitha, and S. Manonmani, “Kinetic study on adsorption of cr (vi), ni (ii), cd (ii) and pb (ii) ions from aqueous solutions using activated carbon prepared from cucumis melo peel,” Applied Water Science, vol. 8, no. 1, p. 36, 2018. https://doi.org/10.1007/s13201-018-0674-1
[39] Y. Yi, J. Lv, Y. Liu, and G. Wu, “Synthesis and application of modified litchi peel for removal of hexavalent chromium from aqueous solutions,” Journal of Molecular Liquids, vol. 225, pp. 28–33, 2017. https://doi.org/10.1016/j.molliq.2016.10.140