Box-Behnken design for optimizing the acid blue dye adsorption on flower wastes
Main Article Content
Keywords
Adsorption, Removal of dyes, Acid blue 9, Alternative adsorbents, Adsorption on Surfaces, Response surface designs.
Abstract
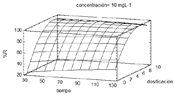
In this paper we identified the best conditions for the removal of Acid Blue 9 dye (AB9) using ower wastes (FW) as an adsorbent were determined using a full factorial 23 and a Box-Behnken design for further optimization. Adsorbent dose (D), dye concentration (C) and contact time (t), were the assessed variables. The dye content was quantied by UV-Vis spectrometry. The statistical model presented an adequate adjustment coecient (R2 = 99,18%), allowing to achieve a removal of 98,5% with a dosage of 7,8 gL-1, dye concentration of 7,11 mgL-1 and contact time of 104 min. These results suggest that owers wastes are an alternative and potential adsorbent material for the treatment of dissolved acid dyes.
PACS: 68.43.-h
MSC: 62K20
Downloads
References
[2] E. Forgacs, T. Cserháti, and Otros, “Removal of synthetic dyes from wastewaters: a review,” Environment international, vol. 30, no. 7, pp. 953–971, 2004.
[3] T. Robinson, G. McMullan, R. Marchant, and P. Nigam, “Remediation of dyes in textile effluent: a critical review on current treatment technologies with a proposed alternative,” Bioresource technology, vol. 77, no. 3, pp. 247–255, 2001.
[4] G. Walsh, L. Bahner, and W. Horning, “Toxicity of textile mill effluents to freshwater and estuarine algae, crustaceans and fishes,” Environt pollut, vol. 21, no. 3, pp. 69–79, 1980.
[5] K. Chung and C. Cerniglia, “Mutagenicity of azo dyes: structure activity relationship,” Mutat. Res, vol. 277, no. 3, pp. 201–220, 1992.
[6] A. Attia, W. Rashwan, and S. Khedr, “Capacity of activated carbon in the removal of acid dyes subsequent to its thermal treatment,” Dyes and Pigments, vol. 69, no. 3, pp. 128–136, 2006.
[7] W. Tsai, C. Chang, and C. Ing, “Adsorption of acid dyes from aqueous solution on activated bleaching earth,” Journal of colloid and interface science, vol. 275, no. 1, pp. 72–78, 2004.
[8] A. Khataee and H. Khataee, “Photooxidative removal of the herbicide acid blue 9 in the presence of hydrogen peroxide: modeling of the reaction for evaluation of electrical energy per order,” Journal of environmental science and health Part. B, Pesticides, food contaminants, and agricultural wastes, vol. 43, no. 7, pp. 562–568, 2008.
[9] N. Daneshvar and A. Khataee, “Removal of azo dye C. I. acid red 14 from contaminated water using fenton , UV/H2O2 , UV/H2O2/ FE (II), UV/H2O2/FE(III), UV/H2O2/FE(III)/ oxalate process: a comparative study,” J Environ Sci Health A Tox Hazard Subst Environ Eng, vol. 41, no. 3, pp. 37–41, 2012.
[10] ASOCOLFLORES, “Guía ambiental para el subsector floricultor colombiano,” 2013, available: http://www.asocolflores.org/ [citado 18 de Febrero de 2013.
[11] M. Rafatulla, O. Sulaiman, R. Hashim, and A. Ahmad, “Adsorption of methylene blue on low-cost adsorbents: a review,” Journal of hazardous materials, vol. 177, no. 1–3, pp. 70–80, 2010.
[12] Z. Aksu, “Application of biosorption for the removal of organic pollutants: a review,” Process Biochemistry, vol. 40, no. 3–4, pp. 997–1026, 2005.
[13] G. Crini, “Non–conventional low–cost adsorbents for dye removal: a review,” Bioresource technology, vol. 97, no. 9, pp. 1061–1085, 2006.
[14] E. Suárez and A. Hormaza, “Estudio del proceso de biosorción de dos colorantes estructuralmente diferentes sobre residuos avícolas,” Revista de la Sociedad Química del Perú, vol. 75, no. 3, pp. 329–338, 2009.
[15] F. Pavan, Y. Gushikem, A. Mazzocato, S. Días, and E. Lima, “Statistical design of experiments as a tool for optimizing the batch conditions to methylene blue biosorption on yellow passion fruit and mandarin peels,” Dyes and Pigments, vol. 72, no. 2, pp. 256–266, 2007.
[16] R. Sivaraj, C. Namasivayam, and K. Kadirvelu, “Orange peel as an adsorbent in the removal of acid violet 17 (acid dye) from aqueous solutions,” Waste management, vol. 21, no. 1, pp. 105–110, 2001.
[17] K. Low, C. Lee, and C. Leo, “Removal of metals from electroplating wastes using banana pith,” Bioresource Technology, vol. 51, no. 2–3, pp. 227–231, 1995.
[18] G. Doria, A. Hormaza, and D. Gallego, “Cascarilla de arroz: material alternativo y de bajo costo para el tratamiento de aguas contaminadas con cromo (vi),” Revista Gestión y Medio Ambiente, vol. 14, no. 1, pp. 73–84, 2011.
[19] A. Hormaza, D. Figueroa, and A. Moreno, “Evaluación de la remoción de un colorante azo sobre tuza de maíz mediante diseño estadístico,” Revista de la Facultad de Ciencias Universidad Nacional de Colombia, vol. 1, no. 1, pp.61–71, 2012.
[20] T. Robinson, B. Chandran, and P. Nigam, “Effect of pretreatments of three waste residues, wheat straw, corncobs and barley husks on dye adsorption,” Bioresource technology, vol. 85, no. 2, pp. 119–124, 2002.
[21] Y. Ho, W. Chiu, and C.Wang, “Regression analysis for the sorption isotherms of basic dyes on sugarcane dust,” Bioresource technology, vol. 96, no. 11, pp. 1285–1291, 2005.
[22] A. Khataee, V. Vatanpour, and A. Ghadim, “Decolorization of c.i. acid blue 9 solution by uv/nano-tio2, fenton, fenton-like, electro-fenton and electrocoagulation processes: A comparative study,” Hazardous Materials, vol. 161, no.2–3, pp. 1225–1233, 2009.
[23] K. Ravikumar, S. Krishnan, S. Ramalingam, and K. Balu, “Optimization of process variables by the application of response surface methodology for dye removal using a novel adsorbent,” Dyes and Pigments, vol. 72, no. 1, pp.66–74, 2007.
[24] M. Elibol, “Response surface methodological approach for inclusion of perfluorocarbon in actinorhodin fermentation medium,” Process Biochemistry, vol. 38, no. 5, pp. 667–673, 2002.
[25] R. Myers, D. Montgomery, and C. Anderson-Cook, Response Surface Methodology: Process and Product Optimization Using Designed Experiments. Wiley, 2009.
[26] F. Tenjo, E. Montes, and J. Matínez, “Comportamiento reciente (2000-2005) del sector floricultor colombiano,” 2013, available http://www.banrep.gov.co/docum/ftp/borra363.pdf [citado 20 de Febrero de 2013].
[27] A. Gómez and X. Tovar, “Elaboración de un abono orgánico fermentado a partir de residuos de flores (pétalos de rosa) y su caracterización para uso en la producción de albahaca,” Tesis de pregrado, Pontificia Universidad Javeriana Colombia, 2008.
[28] A. Echavarría, “Biodegradación en fase sólida de un colorante sintético azo con hongos de podredumbre blanca sobre residuos de flores,” Tesis de pregrado, Universidad Nacional de Colombia, Medellín, 2009.
[29] A. Moreno, D. Figueroa, and A. Hormaza, “Adsorción de azul metileno sobre cascarilla de arroz utilizando un diseño estadístico de experimentos,” Producción más limpia, vol. 7, no. 1, pp. 9–18, 2012.
[30] ——, “Diseño estadístico para la remoción eficiente del colorante rojo 40 sobre tuza de maíz,” Producción más limpia, vol. 7, no. 2, pp. 9–19, 2012.
[31] P. Tripathi, V. Srivastava, and A. Kumar, “Optimization of an azo dye batch adsorption parameters using box-behnken design,” Desalination, vol. 249,no. 3, pp. 1273–1279, 2009.
[32] V. Ponnusami, V. Krithika, R. Madhuram, and S. Srivastava, “Biosorption of reactive dye using acid-treated rice husk: factorial design analysis,” Journal of hazardous materials, vol. 142, no. 1–2, pp. 397–403, 2007.