Critical Constants Correlation from van der Waals Equation
Main Article Content
Keywords
Van der Waals, critical point, equation of state, empirical correlations, group contributions
Abstract
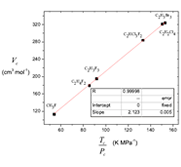
The cubic van der Waals equation of state at the critical condition is reduced to a linear function (Vc vs. Tc /Pc coordinates) with one adjustable parameter. It is shown that at the critical point the relation Vc = 3Vo must not hold as van der Waals suggested, but the attractive constant α = Pc Vc2 remains. Selected values of Tc, Pc, Vc compiled by Ihmels where focused on testing the quality of several empirical equations relating critical conditions. It is shown that the obtained critical constants correlation is a general form of the empirical expressions proposed by Young, Meissner, Bird, Grigoras and Ihmels. From the resulting correlation function, a function for the critical compressibility is proposed. The critical volume Vc and the ratio Tc /Pc have been expressed in group contributions.
Downloads
References
[2] J. D. van der Waals, “Over de continuiteit van den gasen vloeistoftoestand,” Ph.D. dissertation, Leiden, A,W, Sijthoff, 1873. [Online]. Available: https://trove.nla.gov.au/work/31662501
[3] S. Young, “On the law of cailletet and mathias and the critical density,” Proc. Phys. Soc., London, 1899.
[4] J. D. van der Waals. (1912, December) The equation of state for gases and liquids. The Nobel Prize Organisation. [Online]. Available: shorturl.at/uxGR2
[5] S. Velasco, M. J. Santos, and J. A. White, “Consistency of vapor pressure equations at the critical point,” Industrial & Engineering Chemistry Research, vol. 54, pp. 12 993–12 998, 2015. [Online]. Available:
https://doi.org/10.1021/acs.iecr.5b03577
[6] Z. Li, L. Zuo, W. Wu, and L. Chen, “The new method for correlation and prediction of thermophysical properties of fluids. critical temperature,” Journal of Chemical and Engineering Data, vol. 62, no. 11, pp. 3723–3731, 2017. [Online]. Available: https://doi.org/10.1021/acs.jced.7b00454
[7] A. Mersmann and M. Kind, “Prediction of mechanical and thermal properties of pure liquids, of critical data, and of vapor pressure,” Industrial & Engineering Chemistry Research, vol. 56, pp. 1633–1645, 2017. [Online].
Available: https://pubs.acs.org/doi/pdf/10.1021/acs.iecr.6b04323
[8] Cailletet and Mathias, “Recherches sur les densites des gaz liquefies et de leurs vapeurs saturees,” Journal de Physique Theorique et Appliquee, vol. 5, pp. 549–564, 1886. [Online]. Available: https://doi.org/10.1051/jphystap:018860050054900
[9] E. C. Ihmels, “The critical surface,” Journal of Chemical and Engineering Data, vol. 55, no. 9, pp. 3474–3480, 2010. [Online]. Available: http://dx.doi.org/10.1021/je100167w
[10] H. P. Meissner and E. M. Redding, “Prediction of critical constants,” Industrial and Engineering Chemistry, vol. 34, no. 5, pp. 521–526, 1942. [Online]. Available: http://dx.doi.org/10.1021/ie50389a003
[11] R. B. Bird, J. O. Hirschfelder, and C. Curtiss, “Molecular theory of gases and liquids,” Trans. Am. Soc. Mech. Eng., vol. 76, p. 1011, 1954.
[12] S. Grigoras, “A structural approach to calculate physical properties of pure organic substances: The critical temperature, critical volume and related properties,” Journal of Computational Chemistry, vol. 11, pp. 493–510,
1990. [Online]. Available: https://doi.org/10.1002/jcc.540110408
[13] P. J. Mohr, D. B. Newell, and B. N. Taylor, “Codata recommended values of the fundamental physical constants: 2014,” Rev. Mod. Phys., vol. 88, p. 035009, 2016. [Online]. Available:
https://link.aps.org/doi/10.1103/RevModPhys.88.035009
[14] K. A. Kobe and R. E. Lynn, “The critical properties of elements and compounds.” Chemical Reviews, vol. 52, no. 1, pp. 117–236, 1953. [Online]. Available: http://dx.doi.org/10.1021/cr60161a003
[15] J. A. Riddick, W. B. Bunger, and T. K. Sakano, Organic Solvents: Physical Properties and Methods of Purification. Wiley, 1986.
[16] B. E. Poling, J. M. Prausnitz, and J. P. OConnell, The Properties of Gases and Liquids, 5th ed. McGraw Hill, 2004.
[17] K. Denbigh, The principles of Chemical Equilibrium. Cambridge, UK: Cambridge University., 1971.
[18] S. M. Walas, Phase Equilibria in Chemical Engineering. Butterworth-Heinemann, 1985.
[19] C. L. Yaws, Chemical properties handbook: physical, thermodynamics, enviromental, transport, safety, and health related properties for organic and inorganic materials. McGraw-Hill, 1999.
[20] J. O. Valderrama and R. E. Rojas, “Critical properties of ionic liquids. revisited,” Industrial and Engineering Chemistry Research, vol. 48, no. 14, pp. 6890–6900, 2009. [Online]. Available: https://doi.org/10.1021/ie900250g
[21] C. L. Yaws, Thermophysical Properties os Chemicals and Hydrocarbons. Lamar University Beaumont, Texas, Elsevier, 2009.