Correlación de constantes críticas a partir de la ecuación de Van der Waals
Main Article Content
Keywords
Van der Waals, punto crítico, ecuación de estado, correlaciones empíricas, contribuciones por grupo
Resumen
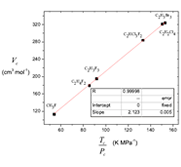
La ecuación de estado cúbica de van der Waals en la condición crítica se reduce a una función lineal (en coordenadas Vc frente a Tc /Pc) con un parámetro de ajuste. Se muestra que en el punto crítico, la constante Vc = 3Vo debe efectivamente descartarse como hizo van der Waals, no así con la constante atractiva α = Pc Vc2. Los valores seleccionados de Tc, Pc, Vc compilados por Ihmels se enfocaron en probar la calidad de varias ecuaciones empíricas renombradas que relacionan condiciones críticas. En este artículo se muestra que la correlación de constantes crítica obtenida es una forma general de estas expresiones empíricas propuestas por Young, Meissner, Bird, Grigoras e Ihmels. Se propone una función para la compresibilidad crítica, única por familia homóloga. El volumen crítico Vc y la relación Tc /Pc se expresa en función de contribución de grupos.
Descargas
Referencias
[2] J. D. van der Waals, “Over de continuiteit van den gasen vloeistoftoestand,” Ph.D. dissertation, Leiden, A,W, Sijthoff, 1873. [Online]. Available: https://trove.nla.gov.au/work/31662501
[3] S. Young, “On the law of cailletet and mathias and the critical density,” Proc. Phys. Soc., London, 1899.
[4] J. D. van der Waals. (1912, December) The equation of state for gases and liquids. The Nobel Prize Organisation. [Online]. Available: shorturl.at/uxGR2
[5] S. Velasco, M. J. Santos, and J. A. White, “Consistency of vapor pressure equations at the critical point,” Industrial & Engineering Chemistry Research, vol. 54, pp. 12 993–12 998, 2015. [Online]. Available:
https://doi.org/10.1021/acs.iecr.5b03577
[6] Z. Li, L. Zuo, W. Wu, and L. Chen, “The new method for correlation and prediction of thermophysical properties of fluids. critical temperature,” Journal of Chemical and Engineering Data, vol. 62, no. 11, pp. 3723–3731, 2017. [Online]. Available: https://doi.org/10.1021/acs.jced.7b00454
[7] A. Mersmann and M. Kind, “Prediction of mechanical and thermal properties of pure liquids, of critical data, and of vapor pressure,” Industrial & Engineering Chemistry Research, vol. 56, pp. 1633–1645, 2017. [Online].
Available: https://pubs.acs.org/doi/pdf/10.1021/acs.iecr.6b04323
[8] Cailletet and Mathias, “Recherches sur les densites des gaz liquefies et de leurs vapeurs saturees,” Journal de Physique Theorique et Appliquee, vol. 5, pp. 549–564, 1886. [Online]. Available: https://doi.org/10.1051/jphystap:018860050054900
[9] E. C. Ihmels, “The critical surface,” Journal of Chemical and Engineering Data, vol. 55, no. 9, pp. 3474–3480, 2010. [Online]. Available: http://dx.doi.org/10.1021/je100167w
[10] H. P. Meissner and E. M. Redding, “Prediction of critical constants,” Industrial and Engineering Chemistry, vol. 34, no. 5, pp. 521–526, 1942. [Online]. Available: http://dx.doi.org/10.1021/ie50389a003
[11] R. B. Bird, J. O. Hirschfelder, and C. Curtiss, “Molecular theory of gases and liquids,” Trans. Am. Soc. Mech. Eng., vol. 76, p. 1011, 1954.
[12] S. Grigoras, “A structural approach to calculate physical properties of pure organic substances: The critical temperature, critical volume and related properties,” Journal of Computational Chemistry, vol. 11, pp. 493–510,
1990. [Online]. Available: https://doi.org/10.1002/jcc.540110408
[13] P. J. Mohr, D. B. Newell, and B. N. Taylor, “Codata recommended values of the fundamental physical constants: 2014,” Rev. Mod. Phys., vol. 88, p. 035009, 2016. [Online]. Available:
https://link.aps.org/doi/10.1103/RevModPhys.88.035009
[14] K. A. Kobe and R. E. Lynn, “The critical properties of elements and compounds.” Chemical Reviews, vol. 52, no. 1, pp. 117–236, 1953. [Online]. Available: http://dx.doi.org/10.1021/cr60161a003
[15] J. A. Riddick, W. B. Bunger, and T. K. Sakano, Organic Solvents: Physical Properties and Methods of Purification. Wiley, 1986.
[16] B. E. Poling, J. M. Prausnitz, and J. P. OConnell, The Properties of Gases and Liquids, 5th ed. McGraw Hill, 2004.
[17] K. Denbigh, The principles of Chemical Equilibrium. Cambridge, UK: Cambridge University., 1971.
[18] S. M. Walas, Phase Equilibria in Chemical Engineering. Butterworth-Heinemann, 1985.
[19] C. L. Yaws, Chemical properties handbook: physical, thermodynamics, enviromental, transport, safety, and health related properties for organic and inorganic materials. McGraw-Hill, 1999.
[20] J. O. Valderrama and R. E. Rojas, “Critical properties of ionic liquids. revisited,” Industrial and Engineering Chemistry Research, vol. 48, no. 14, pp. 6890–6900, 2009. [Online]. Available: https://doi.org/10.1021/ie900250g
[21] C. L. Yaws, Thermophysical Properties os Chemicals and Hydrocarbons. Lamar University Beaumont, Texas, Elsevier, 2009.